Penumbra Jet 7 Xtra Flex Catheter
The Penumbra Jet 7 Xtra Flex Catheter is a medical device that allows doctors to remove blood clots in stroke patients and restore blood flow. Doctors use the catheter in patients who don’t respond to clot-busting drugs. Penumbra issued a recall in December 2020 after reports of serious injury and death.
When blood clots lodge in the blood vessels of the brain, this obstructs blood flow. When blood flow to the brain stops, this causes a stroke. Doctors will first try clot-busting drugs to break up clots and restore blood flow.
But if this doesn’t work, clinicians may recommend a direct aspiration mechanical thrombectomy. In this procedure, doctors use devices such as the Penumbra Jet 7 Reperfusion Catheter with Xtra Flex Technology — also known as the Jet 7 Xtra Flex — to vacuum the clot out of the blood vessel.
These catheters are reperfusion catheters. Reperfusion means to restore blood flow to an organ or tissue, usually after a stroke or heart attack.
Penumbra added Xtra Flex technology to its Jet 7 Reperfusion Catheter with Standard Tip, and the U.S. Food and Drug Administration first cleared the Jet 7 Xtra Flex for sale in June 2019.
How Does the Catheter Work?
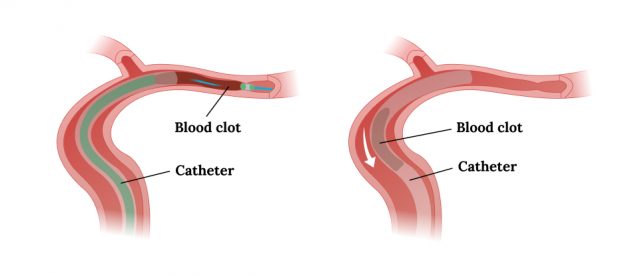
The Jet 7 Xtra Flex catheter and Jet 7Max configuration (Jet 7 Xtra Flex catheter and Max Delivery Device) are a part of the Penumbra System, along with the Penumbra Aspiration Pump and Penumbra Aspiration Tubing.
Patients who suffer an acute ischemic stroke and cannot take clot-busting drugs or do not respond to the drugs are eligible for a mechanical thrombectomy with the Penumbra catheter. Doctors perform the procedure within eight hours of the onset of stroke symptoms.
Before the procedure, the patient will be under general anesthesia or remain conscious but sedated. Doctors insert the Penumbra catheter into a blood vessel in the upper leg and navigate it up through the body until it reaches the clot. In order to navigate and see the catheter in the body, doctors use fluoroscopy — a continuous X-ray beam passed through the body and transmitted to a TV-like monitor.
Once the tip of the catheter comes in contact with the clot, it can be sucked out of the blood vessel and blood flow will be restored.
Complications & Recall
Potential Jet 7 Xtra Flex Catheter adverse reactions and complications are similar to those from Penumbra’s Jet 7 Reperfusion Catheter with Standard Tip.
Complications may occur because of device-related issues or as a result of the procedure. For example, the device may malfunction or some people may have side effects from radiation exposure.
Penumbra’s Risk Information provided on their website doesn’t list the frequency of these adverse events. Patients should make sure they discuss the risks with their doctor.
- Abnormal connection between artery and vein (fistula)
- Allergic reactions to X-ray contrast media
- Blockage in a coronary artery from air bubbles, fatty deposit, broken guidewires or clots (distal embolization)
- Blood clots (thrombosis)
- Blood leakage into surrounding tissues (false aneurysm)
- Blood vessel blockages, damage and spasms
- Brain hemorrhage
- Cataracts, burns, skin reddening or alopecia from radiation exposure
- Contrast media kidney damage
- Death
- Device malfunction
- Failure to remove all of the clot
- Hemorrhage or bruising at catheter access site
- Increased cancer risk or cancer from x-ray exposure
- Infection
- Not enough blood supply to an organ or other part of the body (ischemia)
- Stroke or other neurological problems
Recall
Penumbra issued a recall for all lots of the Penumbra Jet 7 Reperfusion Catheter with Xtra Flex Technology on Dec. 15, 2020. On Jan. 29, 2021, the FDA classified it as a Class 1 — the most serious type of recall where the device may cause serious injury or death.
The recall affects more than 22,600 devices in the United States. The FDA originally cleared the recalled devices through the 510(k) clearance process under K190010 and K191946.
- The Jet 7 Xtra Flex catheter originally cleared on June 16, 2019.
- The Jet 7 Xtra Flex catheter and Max Delivery Device (Jet 7 Max configuration) cleared on Feb. 27, 2020.
Litigation
Lawyers are now accepting cases from stroke patients harmed by the Jet 7 Xtra Flex Catheter and from families who lost loved ones after blood clot removal procedures.
According to the FDA, device failure may lead to death, vessel damage, cerebral infarction, hemorrhage or other serious injury.
People injured by the device may be eligible to file a Jet 7 Xtra Flex Catheter lawsuit against Penumbra and may be entitled to compensation.
5 Cited Research Articles
Consumernotice.org adheres to the highest ethical standards for content production and references only credible sources of information, including government reports, interviews with experts, highly regarded nonprofit organizations, peer-reviewed journals, court records and academic organizations. You can learn more about our dedication to relevance, accuracy and transparency by reading our editorial policy.
- Boisseau, W. et al. (2019). Direct aspiration stroke thrombectomy: a comprehensive review. Retrieved from https://jnis.bmj.com/content/neurintsurg/12/11/1099.full.pdf
- Penumbra. (2020, December 15). Urgent Voluntary Medical Device Recall Notification. Retrieved from https://www.penumbrainc.com/wp-content/uploads/2020/12/JET-7XF-15Dec20.pdf
- U.S. Food and Drug Administration. (2021, January 29). Penumbra’s Urgent Voluntary Recall of JET 7 Catheters with Xtra Flex Technology Due to Increased Risk of Mortality and Serious Injury – Urgent Letter to Health Care Providers. Retrieved from https://www.fda.gov/medical-devices/letters-health-care-providers/penumbras-urgent-voluntary-recall-jet-7-catheters-xtra-flex-technology-due-increased-risk-mortality
- U.S. Food and Drug Administration. (2021, January 29). Penumbra’s Recall of the JET 7 Reperfusion Catheter Due to Distal Tip Damage. Retrieved from https://www.fda.gov/medical-devices/medical-device-recalls/penumbras-recall-jet-7-reperfusion-catheter-due-distal-tip-damage
- Zhang, Y. et al. (2019). General Anesthesia Versus Conscious Sedation for Intracranial Mechanical Thrombectomy: A Systematic Review and Meta‐analysis of Randomized Clinical Trials. Retrieved from https://www.ahajournals.org/doi/10.1161/JAHA.118.011754
Calling this number connects you with a Consumer Notice, LLC representative. We will direct you to one of our trusted legal partners for a free case review.
Consumer Notice, LLC's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
844-420-1914

