JUUL Faces Wrongful Death Lawsuit as Vaping Injuries Surge
Editors carefully fact-check all Consumer Notice, LLC content for accuracy and quality.
Consumer Notice, LLC has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.

The first wrongful death lawsuit for vaping-related lung injuries has been filed against e-cigarette giant JUUL Labs. Inc. in a California federal court.
Daniel David Wakefield’s mother alleges fruity flavors lured the teenager into vaping, and nicotine got him addicted to the habit. According to the lawsuit, Wakefield had vaped for years before dying Aug. 31 at the age of 18. His death was attributed to breathing complications.
Wakefield had started using JUUL e-cigarettes around the age of 15 because of the candy-like flavors, sleek and discreet design, and the representation that the e-cigs were a healthier alternative to traditional cigarettes, according to court documents. He had been exposed to JUUL’s advertising on social media and via direct emails.
Less than a year after he began using JUUL, Wakefield spent three days in the hospital for breathing and lung complications, according to the lawsuit.
“He was so addicted to JUUL that hospital staff affixed nicotine patches to Wakefield’s skin throughout his hospitalization,” the lawsuit states.
Wakefield continued using JUUL after he left the hospital. The morning of his death, his father found him stiff and unresponsive. He had died in his sleep.
“As I have stated before, this lung injury outbreak is serious and we continue to learn of additional deaths being investigated.”
While the Wakefield case is the first wrongful death lawsuit JUUL has faced, another 40 JUUL lawsuits have been combined in a mass litigation in California federal courts. Other injuries alleged in e-cigarette lawsuits include addiction, seizures and strokes.
Health Officials Aim to Clarify Facts About Vaping Dangers
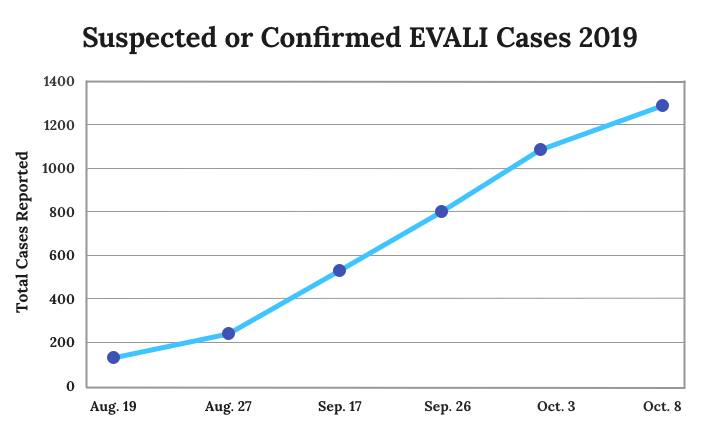
E-cigarette use has been blamed for 26 deaths and 1,299 lung injury cases since health officials first identified a vaping-related outbreak in mid-2019. Public health leaders have named the condition EVALI, or e-cigarette or vaping product use associated lung injury.
The U.S. Food and Drug Administration and the Centers for Disease Control and Prevention along with state health departments are investigating the lung injuries. But months into their probe, investigators still haven’t determined the exact cause of the injuries. The only thing the cases have in common so far is that patients used e-cigarettes.
“As I have stated before, this lung injury outbreak is serious and we continue to learn of additional deaths being investigated,” CDC Principal Deputy Director Dr. Ann Schuchat told reporters during an Oct. 11 telebriefing.
At the same time, public health experts are finding new clues while stressing steps the public can take to protect themselves. Much of their efforts are directed at clarifying what they’ve found and dispelling misinformation.
Vaping-Related Lung Injuries May Not Have ‘Suddenly Happened’
The first news reports of EVALI started appearing in mid-2019, after doctors noticed several similar cases in Wisconsin. By August, federal and state investigators had begun gathering reports from around the country.
The weekly totals of vaping-related injuries include both new patients becoming ill and previously-identified patients that are just now being reported, according to the CDC.
In September, Bloomberg reported it had found 15 documented cases of vaping-related lung injuries dating back to at least 2011. Reporters scouring medical journals found published case studies and medical presentations of vaping injuries similar to EVALI reported in Guam, Japan, Spain, the United Kingdom and the United States all prior to the 2019 outbreak.
Not All Vaping Lung Injuries Involve THC or Vitamin E Acetate
Most, but not all, people whom investigators have talked to after lung injuries reported vaping THC, the active ingredient in marijuana. Vitamin E acetate oil is sometimes added to illicit THC vape fluids as a thickening agent and can cause lung injuries if inhaled.
Both chemicals have been early suspects in the hunt for causes behind the outbreak. The CDC has urged people to avoid THC vaping products and all vaping products “obtained off the street” or from other questionable sources. But investigators have not ruled out nicotine vaping products’ role in EVALI, according to the CDC.
“I think that there will be multiple causes and potentially more than one root cause,” Schuchat said. It’s important to say that we cannot exclude the possibility that nicotine containing products may have some role.”
As of Oct. 11, more than half of EVALI patients questioned said they had vaped nicotine products, either exclusively or along with THC. About 13 percent reported vaping only nicotine.
“I think that there will be multiple causes and potentially more than one root cause.”
The FDA has gathered more than 725 samples of vaping products involved in lung injury cases so far. Vitamin E acetate turned up in 47 percent of the first 225 THC vaping samples tested.
“We have said all along that we are finding vitamin E acetate in some but not all of the THC products,” Mitch Zellner, Director of the FDA’s Center for Tobacco Products said during the telebriefing.
The Vaping Injury Toll Is Not Slowing Down
The number of EVALI cases has grown steadily since federal investigators began looking at the problem.
“We are not seeing a meaningful drop-off in new cases,” Schuchat said. “Unfortunately, many more people have been hospitalized with lung injury each week since we first advised the public about the national outbreak.”
The CDC reported a 52-percent surge in reported cases in a single week in September. Reports have indicated consistent increases ever since, though no uptick has been quite as drastic.
FDA Has Never Approved a Single E-Cigarette for Sale in the U.S.
The FDA was given authority to regulate e-cigarettes and other vaping products in 2016, but it has not yet approved any device currently sold in the U.S.
Makers of devices that were on the market as of Aug. 8, 2016, have to apply for FDA approval by May 12, 2020, to continue marketing them in the U.S.
It wasn’t until October 2019 that the FDA received the first e-cigarette application, according to multiple media reports. Reynolds American sought FDA review of its Vuse e-cig. Vuse had already been on the market for more than six years.
Reynolds is a unit of British American Tobacco and makes Camel and Newport tobacco cigarettes.
There’s No Proof that Vaping Is Safer than Smoking, FDA Says
The FDA concedes it may be safer for smokers already addicted to nicotine to completely switch from tobacco to e-cigarettes. But it warns there’s not enough evidence to know for sure.
The agency says researchers are still learning about potential risks of vaping. Research shows that several dangerous chemicals in tobacco smoke are also found in the vapor from e-cigs, according to the FDA.
"The law is clear that, before marketing tobacco products for reduced risk, companies must demonstrate with scientific evidence that their specific product does in fact pose less risk or is less harmful."
The agency fired off a warning letter to JUUL Labs in September 2019 saying it was illegal for the company to claim its JUUL e-cigarettes were safer than smoking tobacco.
“[T]he law is clear that, before marketing tobacco products for reduced risk, companies must demonstrate with scientific evidence that their specific product does in fact pose less risk or is less harmful,” the letter read.
E-cigarettes and their fluids are still classified as tobacco products under federal regulations.
“There is no safe tobacco product,” Schuchat said. “The use of any tobacco products, including e-cigarettes, carries a risk.”
FDA: E-Cigarettes Are Not Approved to Help You Quit Smoking
E-cigarettes are not approved as aids to help people quit smoking, according to the FDA.
Manufacturers have to submit proof to the agency that their devices are safe and effective to be approved as a method to help people stop smoking. The FDA warns that e-cigarettes may expose users to some of the same toxic chemicals found in combustible cigarette smoke.
The agency has approved several smoking cessation aids that contain nicotine. Over-the-counter versions come in patches, gum and lozenges.
Nicotrol is the only FDA-approved prescription nicotine replacement therapy. The nicotine-containing product is available as a nasal spray or oral inhaler.
“If adults are using e-cigarette or vaping products to quit cigarette smoking, they should not return to smoking cigarettes.”
The agency has approved two smoking cessation products that do not contain nicotine: Chantix (varenicline tartrate) and Zyban (buproprion hydrochloride). Both are tablets and available by prescription only.
The CDC recommends that people who are using e-cigs to stop smoking instead try approved and proven methods if they are worried about vaping-related lung injuries.
“If adults are using e-cigarette or vaping products to quit cigarette smoking, they should not return to smoking cigarettes,” Schuchat said. “We recommend they use evidence-based guidance.”
Schuchat said people trying to break a THC vaping habit also have resources available through the Substance Abuse and Mental Health Services Administration’s National Helpline. The toll free number is 1-800-662-4357.