Exactech Announces Equinoxe Shoulder System Recall
Editors carefully fact-check all Consumer Notice, LLC content for accuracy and quality.
Consumer Notice, LLC has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
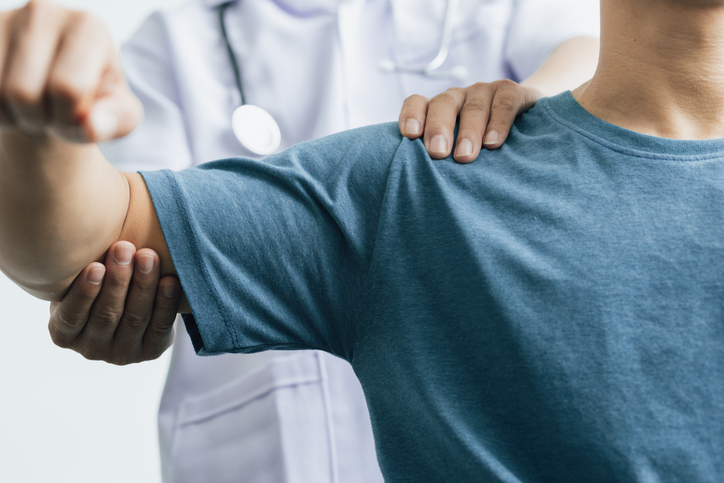
Exactech initiated a voluntary recall this week for its Equinoxe Shoulder System joint replacement devices, the latest action in response to ongoing issues with defective packaging.
The recall affects devices packaged between 2004 and August 2021 and were sealed in bags that lacked an essential oxygen barrier that protects from oxidation. According to the U.S. Food & Drug Administration, this can cause the plastic components to degrade, leading to faster wear, failure, cracking or fracture.
“If your Equinoxe Shoulder System is functioning well and you have no pain or symptoms, the FDA does not recommend surgery to remove a well-functioning device,” the FDA stated.
The Agency further advises Equinoxe Shoulder System patients experiencing symptoms like pain, swelling, grinding or weakness near the implant to contact their health care providers.
In January the FDA issued a safety communication to patients and health care providers warning of the issues after Exactech failed to issue a recall. In March the company issued a letter to surgeons regarding the FDA notice, but it wasn’t until April 19 that it announced the recall and plans to remove the unused inventory.
Legal Repercussions and Ongoing Litigation
Exactech’s defective packaging has caused massive recalls of its other products including knee, ankle, shoulder and hip devices. The firm recalled thousands of its medical devices in 2021 and 2022 because of its defective packaging.
The issue has already sparked more than 1,400 Exactech lawsuits filed by patients who are seeking compensation for injuries. Lawsuits claim the defects have caused pain, stiffness, loosening, device failure that results in revision surgery, limited range of motion and osteolysis (bone loss).
The lawsuits are consolidated into multidistrict litigation in the U.S. District Court for the Eastern District of New York and there have been no trials. Lawyers are still taking clients in these cases.
What Should Impacted Patients Do?
Defective Exactech packaging could cause problems over time for devices that include early and excessive device wear, component fracture, device failure, new or worsening pain, bone loss, swelling in the affected area or revision surgery as a result of these issues, according to the FDA.
Patients who have implanted Equinoxe Shoulder System joint replacement devices manufactured by Exactech between 2004 and August 2021 are urged by the FDA to check their device against the Unique Device Identifier table provided by Exactech.
Each medical device sold in the U.S. has a unique device identifier.
“The UDI is a unique numeric or alphanumeric code that generally includes a Device Identifier (DI) that identifies the labeler and the specific version or model of a device, as well as a Production Identifier (PI) that identifies additional information, which may include lot number, serial number, expiration date and manufactured date,” according to the FDA.
Anyone who believes they had a problem with their device can report it through the MedWatch Voluntary Reporting form or call 1-800-332-1088 for more information on how to mail or fax the form.